Well, time has flown since we sampled the first household in the 99 households study. On 7th June we visited our 66th household, meaning that after 8 months we are now two thirds of the way through. The project is taking us to all parts of Nairobi, as the maps illustrate. The field teams normally spend Monday to Wednesday collecting data, then use Thursdays and Fridays to recruit new households to the study, meet with local chiefs and county officials, give feedback to participants and keep on top of all the other jobs, such as vehicle maintenance, stock-keeping, accounting and paperwork. The wildlife team regularly go out on evenings and weekends to set and check traps for rodents and bats (who inconveniently refuse to venture out during normal working hours!) In some areas it has occasionally been necessary to conduct the study interviews in the evening, when participants return from work. Having to be flexible to fit around our human and animal participants’ needs, plus the perennial problem of Nairobi traffic, means early starts and long days.
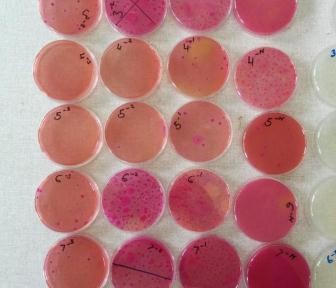
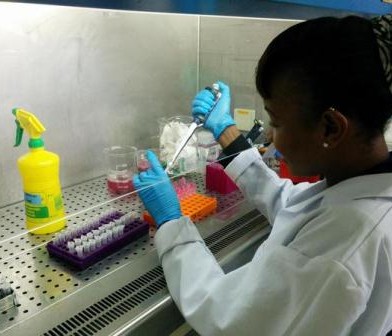
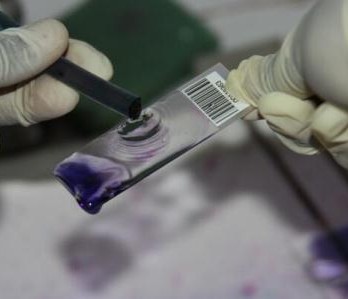
The laboratory teams also come in for their share of hard work. Even with motorbike couriers, samples normally do not arrive at the labs until the afternoon, especially when large households are sampled. To process all these samples takes time. Each sample is first incubated in an enrichment broth, then undergoes two rounds of purification on a special type of agar which selects for E. coli, before being cultured on a more general agar prior to freezing the bacteria for storage. As you may imagine, this is several days’ work – each step takes at least 24 hours – and of course the bacteria don’t stop growing at weekends! Timing of steps is crucial, to ensure that pure colonies can be selected for storage. Later on, batches of isolates are revived and a number of biochemical tests are performed, to check that the bacteria we send to the UK for sequencing really are the E. coli that we are interested in. Once we are reasonably sure that what we have is an E. coli, they have to be regrown once more so that they can be sent to ILRI, where the DNA is extracted to send to the sequencing facility at Oxford.
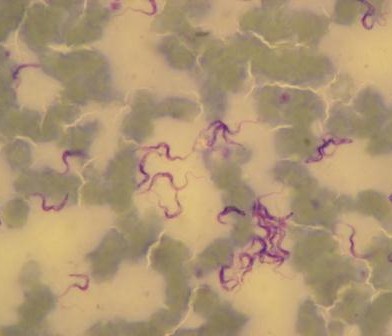
So as you might imagine, it is extremely gratifying to finally start to see some of the results of all this hard work. Dr. Melissa Ward recently visited the teams in Nairobi and brought with her some of the first outputs of the sequencing to show us. In return, we took her along to see the sampling in action, in one of the slum sites. Melissa said, “It really brings the project to life, to see exactly how all the data and the samples are collected. Now, when I sit at my computer, I can really understand where it’s all coming from.” For us, it was equally exciting to get some tantalising glimpses of what the final dataset might look like and what kind of patterns we may be able to identify from the phylogenetic structure and genomic data. We’re not giving anything away at this stage – but we can tell you that we definitely have E. coli – and lots of it!
 Article by Judy Bettridge
Article by Judy Bettridge



 Under the
Under the 
 Human, food and environmental data are among the wide range of data collected within the
Human, food and environmental data are among the wide range of data collected within the 

 As we approach the final quarter of the
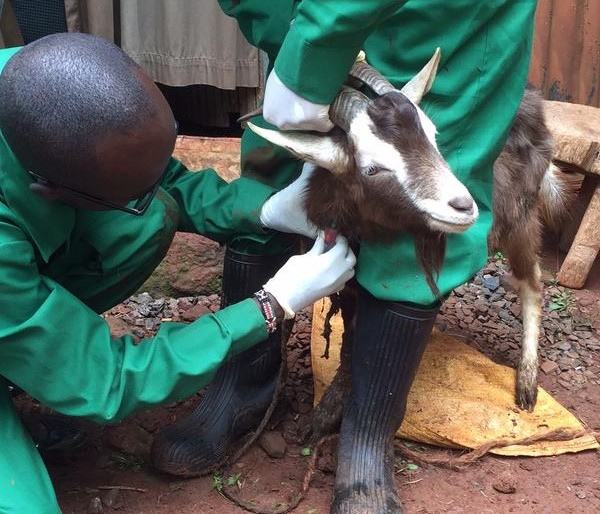
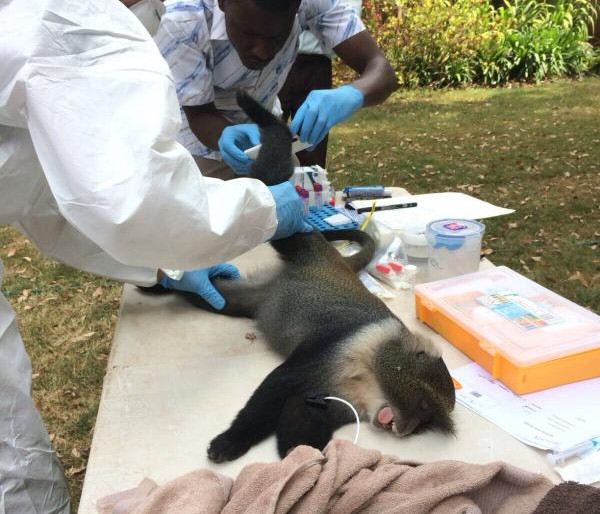
As we approach the final quarter of the  disease ecologists, and additional samples provide an important resource for future epidemiological work. We next check the rodent traps – we use live-capture Sherman traps which are set throughout the house, livestock-keeping facilities and the household compound. Any rodents that we catch are transported back to the lab at ILRI, where they are humanely euthanized and subjected to a post-mortem examination (PME). This procedure is used to permit the collection of fresh faeces and organ samples which are stored frozen and in formalin. The latter ensures that tissues from these animals are preserved for histopathological interpretation, should the need arise in the future. As dusk settles over Nairobi, the sampling team heads back to the house to trap bats.
disease ecologists, and additional samples provide an important resource for future epidemiological work. We next check the rodent traps – we use live-capture Sherman traps which are set throughout the house, livestock-keeping facilities and the household compound. Any rodents that we catch are transported back to the lab at ILRI, where they are humanely euthanized and subjected to a post-mortem examination (PME). This procedure is used to permit the collection of fresh faeces and organ samples which are stored frozen and in formalin. The latter ensures that tissues from these animals are preserved for histopathological interpretation, should the need arise in the future. As dusk settles over Nairobi, the sampling team heads back to the house to trap bats. unharmed. When we encounter a bird or bat roost, we use tarpaulins spread underneath the roost in order to collect pooled faecal samples representative of the individual animals using the roost.
unharmed. When we encounter a bird or bat roost, we use tarpaulins spread underneath the roost in order to collect pooled faecal samples representative of the individual animals using the roost.
 Article by
Article by 



































You must be logged in to post a comment.