Integrated studies on the emergence of zoonoses in urban settings
Webinar: Integrated studies on the emergence of zoonoses in urban settings
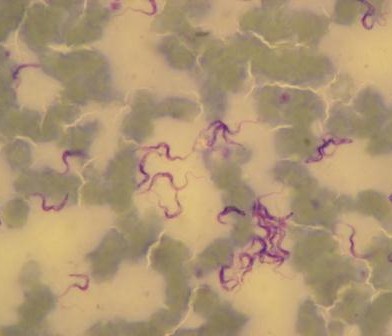
Nairobi is an exemplar of a modern sub-Saharan metropolis, intimately connected to its hinterland and experiencing rapid population growth. This growth drives the creation of complex human, animal and environmental interfaces, which present risks for the emergence of pathogens that spread between animals and people. In this lecture, our interdisciplinary approach to understanding this complexity will be outlined, illustrating the value in integrating planning, biology, food system economics, pathogen genetics and public health.

Speaker profile

Eric Fèvre is Jointly appointed between the University of Liverpool where he is Professor of Veterinary Infectious Diseases and the International Livestock Research Institute in Kenya. He manages a range of field-orientated projects researching disease transmission and control at the interface between animals and human beings. His research team is a grouping of epidemiologists, ecologists, biologists, veterinarians and medical practitioners interested in the biology and control of (re-)emerging diseases. The group conducts field studies to acquire a wider understanding of pathogen epidemiology to inform policy on optimal and cost-effective methods of disease control. He is an epidemiologist whose work is focussed on understanding the interface between humans, other animals and the environment, and understanding the mechanisms and risks of disease transmission across this interface. He has been working with colleagues at the DPU since 2013.